CDC Scandal: Committee that Withdrew Recommendation for Nasal Flu Vaccine Now Recommends it to Experiment on American Public
 Image from AstraZeneca website on FluMist.
Image from AstraZeneca website on FluMist.
by Brian Shilhavy
Editor, Health Impact News
In June of 2016 the Center for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) admitted that the live attenuated nasal influenza vaccine known as “FluMist” was not effective, and was not recommended for the 2016-2017 flu season. It was also not recommended for the current flu season (2017-2018).
The CDC's own data showed that the nasal vaccine was not effective. The CDC press release in 2016 stated, “This three percent estimate means no protective benefit could be measured.”
Shortly after this announcement in 2016, a family in Utah went public with their story, explaining how their 8-year old daughter died from influenza, even though she had been vaccinated with FluMist. They had trusted the CDC and their flu recommendations, but now they have lost their daughter.
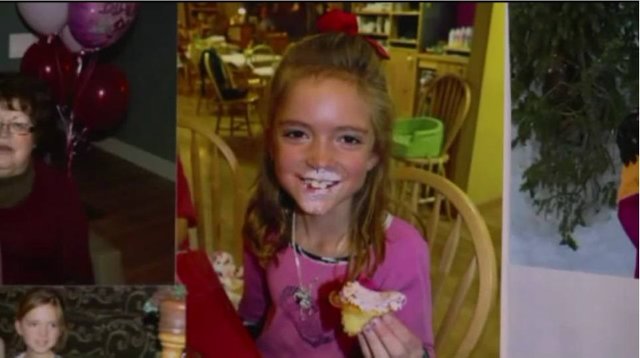 Mackenzie Coyne was vaccinated against the flu with FluMist, but died from flu complications. Image source courtesy Fox13 Salt Lake City.
Mackenzie Coyne was vaccinated against the flu with FluMist, but died from flu complications. Image source courtesy Fox13 Salt Lake City.
See:
CDC Admits Failure of Flu Vaccine – Vaccinated People Die of Influenza
Even though the CDC withdrew their recommendation for the failed nasal flu vaccine, FluMist retained its FDA approval and has been available to purchase the past two years.
On February 21, 2018, the CDC's ACIP reversed its decision on FluMist, and voted 12-2 to add FluMist to the CDC’s list of recommended vaccines for the 2018-2019 influenza season.
The CDC Admits They do not Know if the New Version of FluMist is Effective, and That They Need to Test it on the American Public to Find Out
The CDC ACIP has apparently NOT recommended the nasal flu vaccine because now it is all of a sudden deemed to be effective.
Stat News reports how members of the ACIP expressed concerns about deciding to reverse their decision and recommend it:
The motion to recommend FluMist passed by a surprising 12-to-2 vote, the outcome of which belies the unease that a number of members of the committee clearly felt about the decision they were making.
They faced, in essence, a Catch-22: It has been impossible to generate the type of data that would normally be used to make a decision like this.
The only way to see if the problem has been solved is to use the vaccine. But without an ACIP recommendation, the vaccine’s use in the U.S. would remain minimal.
“The effectiveness of this formulation against [H1N1] is not known, and is likely to remain unknown until the next H1N1 predominant season, presuming adequate uptake of vaccine. We can’t predict when this will occur,” the CDC’s Dr. Lisa Grohskopf, who presented the report of the ACIP’s influenza working group, told the committee.
So the fact is that the effectiveness of FluMist is still not known, but the CDC has no qualms about testing it on the American public to find out.
One of the doctors on the ACIP panel who voted against recommending FluMist, Henry Bernstein, MD of the Zucker School of Medicine at Hofstra/Northwell in Hempstead, NY, reportedly stated:
I’m a little concerned about whether it would be interpreted that we’re compromising our interpretation of the science.Others also expressed concern about recommending a flu vaccine with no scientific data to support its claims:
The group grappled with how to proceed, with several voting members — and nonvoting members representing groups like the American Academy of Pediatrics and the National Association of Pediatric Nurse Practitioners — voicing concern about a recommendation a number felt would confuse both the public and health care providers who administer flu vaccines.
... members of the committee expressed concern that restoring FluMist to the recommended vaccines list before there is evidence to prove the H1N1 problem has been fixed could further undermine the battered reputation of flu vaccines, especially if it turns out that the change MedImmune made did not improve the vaccine’s performance.
Why is this Defective Pharmaceutical Product Still Approved by the FDA?
As noted above, even though the CDC removed its recommendation of FluMist, it continued to be sold and administered in the United States because it is still approved by the FDA.
One doctor who has sounded the alarm on the fraud behind flu vaccines and explains that the FDA and CDC do not follow its own laws as it is applied to other pharmaceutical products when approving flu vaccines, is Dr. Mark Geier.
Dr. Geier is NOT anti-vaccine. He is an MD and has a PhD in genetics. He spent 10 years working at the National Institute of Health, and was a professor at Johns Hopkins University as a geneticist. He is also the author of over 150 peer-reviewed publications.
He worked on vaccine safety and efficacy for more than 30 years. He was one of four scientists who worked to replace the DTP vaccine, a vaccine that caused every child to become sick with a high fever at the time of vaccination, with the DTaP vaccine, which is an attenuated vaccine and causes illness due to fever in only 3% of those vaccinated.
In the video above, he explains that the flu shot causes Guillain-Barré Syndrome, and that the flu shot is not very effective in preventing the flu.
He also explains that the CDC does not follow the law for vaccines in requiring long-term safety testing for the influenza vaccine like they do with other vaccines, as it is impossible to test a vaccine that changes every year.
So the flu vaccine is basically an experimental vaccine that they want to give out to 300 million people every year. There are also no studies showing the safety of giving the flu vaccine to the same person every single year. However, Dr. Geier points out that the CDC is in the business of distributing flu vaccines, because they represent 300 million doses per year, whereas all the childhood vaccines together only number 20 million.
Dr. Geier goes on to explain that flu is “the wrong thing to vaccinate against” because you have to keep re-vaccinating against it every year, unlike childhood infectious diseases, such as smallpox, that are only vaccinated for once. Dr. Geier points out how ridiculous it is spend billions of dollars on a vaccine that might, at its best, save about 50 lives a year, when there are far more serious problems causing death that are more worthy of that kind of expenditure.
Facts About the Dangerous Live-Attenuated Nasal Flu Vaccine that the Public is Largely Unaware About Prior to Receiving this Flu Vaccine
Flu vaccines in the United States are administered so routinely, that the public is largely unaware of its risks and dangers.
The nasal flu vaccine FluMist is currently the only flu vaccine that is not injected into your muscle, but is sprayed into your nasal passage.
It is what is termed a "live attenuated nasal flu vaccine," as opposed to other flu shots that do not contain live viruses.
Therefore, warnings are given regarding the nasal flu vaccine because for 7 days after receiving the vaccine it is possible to pass the flu viruses contained in the vaccine on to others.
However, these warnings are not commonly known or advertised.
One place this warning is documented is the Vaccine Information Statements (VIS), which by federal law is supposed to be presented to you prior to making a decision about receiving a flu vaccine (or any other vaccine for that matter).
The VIS for the Live, Intranasal flu shot is found here. It clearly states that some people should NOT receive this vaccine, and lists the warnings:
Some people should not get LAIV because of age, health conditions, or other reasons. Most of these people should get an injected flu vaccine instead. Your healthcare provider can help you decide.Tell the provider if you or the person being vaccinated:
• have any allergies, including an allergy to eggs, or
have ever had an allergic reaction to an influenza
vaccine.
• have ever had Guillain-Barré Syndrome (also called
GBS).
• have any long-term heart, breathing, kidney, liver, or
nervous system problems.
• have asthma or breathing problems, or are a child who
has had wheezing episodes.
• are pregnant.
• are a child or adolescent who is receiving aspirin or
aspirin-containing products.
• have a weakened immune system.
• will be visiting or taking care of someone, within the
next 7 days, who requires a protected environment (for
example, following a bone marrow transplant)Sometimes LAIV should be delayed. Tell the provider if
you or the person being vaccinated:• are not feeling well. The vaccine could be delayed
until you feel better.
• have gotten any other vaccines in the past 4 weeks.
Live vaccines given too close together might not work
as well.
• have taken influenza antiviral medication in the past
48 hours.
• have a very stuffy nose.
Part of the information that is required by federal law to be given to everyone prior to receiving the flu vaccine is given in this statement from the VIS:
If you have any severe, life-threatening allergies.If you ever had a life-threatening allergic reaction after a dose of flu vaccine, or have a severe allergy to any part of this vaccine, you may be advised not to get vaccinated. Most, but not all, types of flu vaccine contain a small amount of egg protein.
Notice that the only potential allergen given in this warning is to “egg protein,” yet the warning is for “any part of this vaccine.”
Likewise, the manufacturer of FluMist states on their website:
Who should not get FluMist Quadrivalent?
You should not get FluMist Quadrivalent if you have a severe allergy to eggs or to any inactive ingredient in the vaccine; have ever had a life-threatening reaction to influenza vaccinations; or are 2 through 17 years old and take aspirin or medicines containing aspirin – children or adolescents should not be given aspirin for 4 weeks after getting FluMist Quadrivalent unless your healthcare provider tells you otherwise.Children under 2 years old have an increased risk of wheezing (difficulty with breathing) after getting FluMist Quadrivalent.
(The full list of side effects, ingredients and warnings can be found here, and takes up a couple of pages.)
How many people are given a list of ingredients in FluMist, or any other flu vaccine, prior to being injected, to know if they might have an allergic reaction?
For example, did you know that one of the new flu vaccines on the market contains armyworms and insects?
Here is the list of ingredients for FluMist per the package insert:
What are the ingredients in FluMist Quadrivalent?Any of the inactive ingredients could cause an allergic reaction. "Gentamicin," for example, is an antibiotic, and "monosodium glutamate" is better known as MSG, a common food additive with side effects.Active
Ingredient: FluMist Quadrivalent contains 4 influenza virus strains that are weakened (A(H1N1), A(H3N2), B Yamagata lineage, and B Victoria lineage).Inactive
Ingredients: monosodium glutamate, gelatin, arginine, sucrose, dibasic potassium phosphate, monobasic potassium phosphate, and gentamicin.
Look up each ingredient and search the known side effects prior to making a decision about having this vaccine sprayed into your nasal passage, or the nasal passage of your child.
Standing Orders: Mass Vaccination Goals Routinely Ignore Federal Laws for Flu Vaccination
 Image from Walgreens.com
Image from Walgreens.com
Flu shots are advertised to the American public as simple procedures with no risks or side effects, and one can walk into a local drugstore and get a flu shot as easily as buying a candy bar.
However, as I have just shown, there are actually federal laws in place that require the consumer/patient to be fully informed of the risks of the flu vaccine, particularly FluMist since it is a live-attenuated nasal spray that can potentially infect others for up to 7 days.
The fact that health care facilities are firing nurses and other employees who refuse the flu vaccine as a requirement of employment, but have no policy in place for those who receive live-attenuated vaccines, suggests that health care administrators are not even aware of these federal requirements, or don't care.

In evidence of the attitude of "don't care" when it comes to following federal laws for administering flu vaccines, we can look at one initiative by a vaccine manufacturer to get more people vaccinated called: Using Standing Orders to Vaccinate Adults
"Standing Orders" are a way to get more adults vaccinated by loosening the restrictions in place that were originally intended to protect patients and their right to consult with their physicians about medical decisions.
From the website, which is sponsored by Pfizer:
Standing orders authorize nurses and other appropriately trained health care personnel, where allowed by state law, to assess a patient’s immunization status and administer vaccinations according to a protocol approved by an institution, physician, or other authorized practitioner. Immunization standing orders work by enabling assessment and vaccination of the patient without the need for clinician examination or direct order from the attending provider at the time of the interaction. (emphasis added)
These "Standing Orders" apparently authorize a broader range of personnel who can administer vaccines. Studies done in the past on the impact of Standing Orders, such as with the HPV vaccine Gardasil, show that the use of these Standing Orders does increase vaccination rates.
The Immunization Action Coalition, which works with Pfizer to educate medical professionals about Standing Orders, hosts conferences around the country on how to increase the use of these Standing Orders.
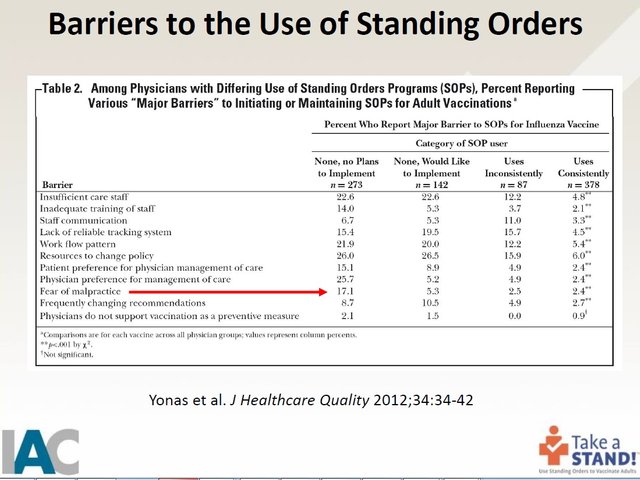
One of the things they teach is that one of the "barriers" to using Standing Orders is the fear of being sued for malpractice. Here is a slide from their presentation:
In order to overcome this barrier to the "fear of malpractice," they educate personnel on the National Vaccine Compensation Program, which was the result of the 1986 law giving legal immunity to the medical community for injuries and deaths resulting from vaccines.
Here is a slide from their presentation:
For more information, see:
The Vaccine Cartel: Largest Criminal Organization in the World – Why Your Flu Shot is Probably Illegal
The CDC is Not Protecting Public Health - Do Your Own Research
The CDC is the largest purchaser of vaccines in the U.S., spending more than $4 BILLION annually of taxpayer funds to purchase and distribute vaccines. There is an obvious conflict of interest, and it is obvious that the ACIP's recommendation to include FluMist for the next flu season is not in the best interest of the public, but instead is in the best interest for the manufacturer of FluMist, who has allegedly threatened to pull the vaccine from the market unless the CDC approves it.
Do your own research, and know the risks. Your doctor may be ignorant or even be constrained by corrupt medical practices preventing him or her from disclosing to you all the facts regarding FluMist or any other flu vaccine, so you need to do your own research.
Here at Health Impact News and VaccineImpact.com we will do our best to present the facts and data you need to make an informed decision regarding the flu vaccine or any other vaccine approved by the FDA and recommended by the CDC.
Originally published on VaccineImpact.com and Health Impact News