Medical research and clinical trial has bridged to Blockchain technology through TRIALL
CHALLENGES IN CLINICAL RESEARCH INDUSTRIES

Clinical trial is really cumbersome and there are inefficient resources to carry out the clinical trials. Most clinical research industries are responsible for the delivery of some medical solutions around the world but on the process they lack sufficient resource to carry out efficient process in clinical research.
When there is a disease out-brake or a pandemic like COVID-19, there will be need for a steady and efficient supply of vaccines, therapeutics, and other innovative medical solutions and it will be so critical in meeting the society’s current and future medical needs. Never the less, the clinical industry will faces several persistent problems that make clinical trial operations to be so cumbersome and resource-inefficient. The clinical research industries face serious problem, for example;
1). Fragmentation, leading to information and data being scattered across
sites and systems.
2). Lack of oversight which is reported by virtually all
clinical trial professionals.
3). Record keeping failure that may caus significant delays,
costs, and safety risks.
4). Data integrity issues is a growing concern according
to authorities globally.
ABOUT TRIALL INDUSTRY
Triall is a blockchain industry that brings Web 3.0 to medical research, create a digital ecosystem of blockchain-integrated software solutions to secure and streamline the development of new vaccines and therapeutics during clinical trials.
The blockchain infrastructure of this industry makes clinical trial data tamper-resistant. It also enables secure and efficient integrations between the many isolated systems and parties involved in clinical trial processes.
This industry's (triall) software is created by clinical trial professionals to ensure optimal user experience, solving actual industry pain points.
SOLUTIONS OFFERED BY TRIALL INDUSTRY

Clinical trials have become increasingly complex and data-heavy. As a result, the number of data points collected per clinical trial has grown significantly over the past decade.
Triall develops blockchain-enabled tools to establish verifiable proof of the existence, integrity, and authenticity of clinical trial data
Triall uses a world-class blockchain and Self-Sovereign Identity (SSI) technologies to secure clinical trial data, and to improve efficiency, quality, trust, and regulatory compliance for all parties involved. This industry increases digital and decentralized approach to clinical trials.
This project also enables secure and efficient connections between the many isolated systems and parties involved in clinical research.
ABOUT TRL TOKEN (TRIALL)

TRL is a utility token that serves as an internal fuel powering the TRIALL Industry. The coin is used to aquire clinical or medical resources or data in the industry, it is also used to pay for medical services and more. $TRL is traded across multiple exchange platforms, and also exists both as an ERC-20 token on the Ethereum network, and as a BEP-20 token on the Binance.
The token ($TRL) will provide access to all current and future Triall solutions. The token facilitates fair and equitable sharing of benefits and access among the stakeholders in the ecosystem by enabling P2P compensation, self-governance, and community engagement. It helps the team to lay a groundwork for a self-sustaining ecosystem, in which a community of clinical researchers, software developers, and other community members are incentivized to create and capture utility and value.
Smart Chain.
TOKEN TYPE:
BEP-20
DECIMALS:
18
CONTRACT ADDRESS:
0xe2eb47954e821dc94e19013677004cd59be0b17f
TOKEN TYPE:
ERC-20
DECIMALS:
18
CONTRACT ADDRESS:
0x58f9102bF53Cf186682Bd9a281d3Cd3c616eEc41
You can buy $TRL on your preferred exchange as explained in this 👉👉 https://medium.com/triall-foundation/triall-and-ferrum-network-launch-a-eth-bsc-bridge-for-trl-e10de2ad463a

Triall gives user the opportunity of investment to Buy and Stake the $TRL 👉 https://www.triall.io/staking/
GOAL OF TRIALL CLINICAL INDUSTRY
By veture of the great team behind this project, the goal is to accelerate the introduction of safe and affordable vaccines and therapeutics into society by streamlining clinical trials. This is the only blockchain that has thought of these idea through the development of blockchain technology, all thanks to the team.
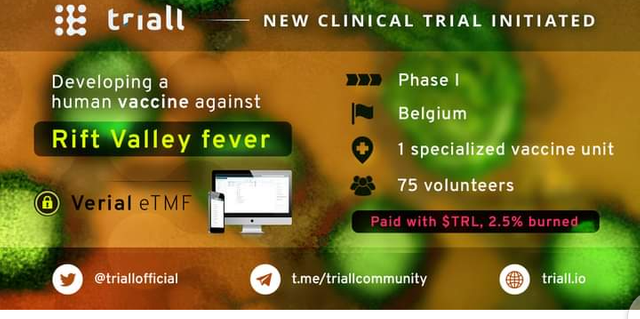
NEW CLINICAL TRIAL INITIATED IN THE TRIALL INDUSTRY.

The awaited blockchain-integrated document management solution Verial eTMF of this industry is now officially in use in a COVID-19 vaccine study.
Details of the study:
🔹 Therapeutic area: COVID-19
🔹 Phase: 1
🔹 Type: Vaccine
🔹 Country: South Africa
🔹 Number of sites: 1
🔹 Number of participants: 45
To promote the global fight against COVID-19, Triall offers an application Verial eTMF for free for this study.
CONCLUSION

The team behind this project has managed over 100+ clinical trials across 30+ countries, and successfully grown companies both in the Life Sciences and Software/Blockchain-as-a-Service industries. The team has also published 250+ peer-reviewed papers on innovation in medical research, featured in top-tier scientific journals such as Nature and Science.
Visit the links below for more information.
👉 https://www.triall.io/
👉 https://t.me/triallcommunity
👉 https://linkedin.com/company/triallofficial
👉 https://twitter.com/triallofficial