Math Tricks Episode 1: Radioactive Decay & First-Order Kinetics
Hello Math Lovers! Welcome to my brand new series called "Math Shortcuts". If you are preparing for board examination or just want to learn some cool math tricks, then you came to the right place.
Examinations can be stressful and overwhelming especially when faced with a test questionnaire filled with hundreds of problems you need to solve within few hours. But don't worry, today I will teach you some tricks how to solve difficult problems within seconds. That's right! With your calculator, you can skip the complicated math and get the right answer! So without further ado, let's start!

If you have an idea, try to solve this problem first without looking at the solution below, then check your answer later!
Long Method: (What they teach you in school)
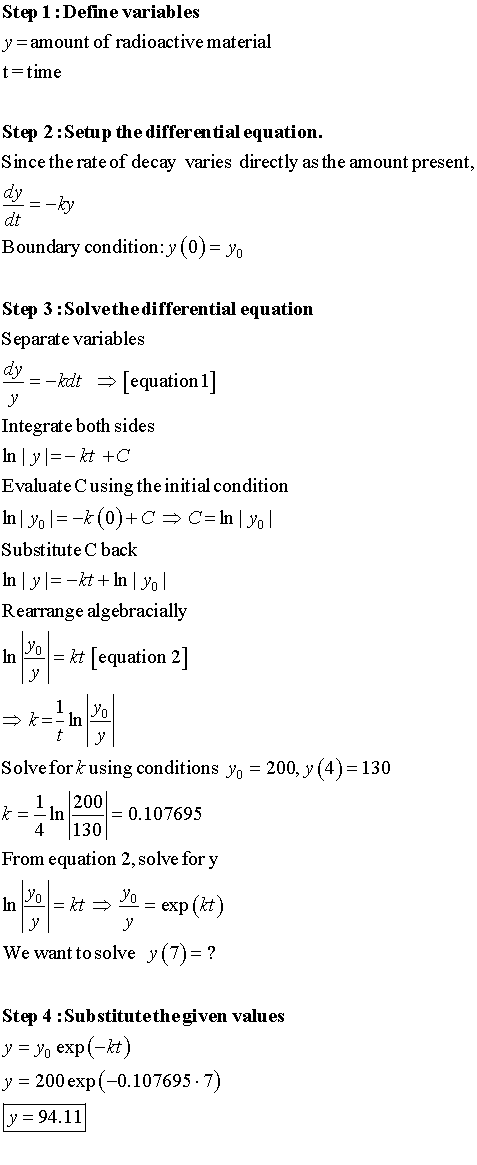
If you want a detailed explanation, you can watch this video that I created.
Short Method : (What they don't teach you at school)
Now, what is the trick to solve this problem in a few seconds? Here's the secret recipe.
So the answer is option B (94.11 g). Did you get the right answer?
What's great is that you can actually use this technique to similar problems such as bacterial growth and decay, first-order chemical kinetics, and any problem where it says "the rate varies directly as amount present". If you use this technique, you can save a lot time and get your test done in no time! However, if the problem doesn't say "first-order kinetics" or "rate depends on amount present", you need to use another method, which we will talk about in the next post.
Exercise
1.) The growth rate of certain strain of bacteria is found to vary directly with amount of bacteria present. If an initial population of 500 doubles within 2 hours. How many bacteria will be there after 3 hours?
a.) 1414 b. 1515 c.) 1616 d.) 1717
2.) A certain chemical reaction follows a first order kinetics. 20 g of reactant is added into the mixture and after 10 minutes, its concentration reduced to 40% of its initial value. How long will it take for the concentration to reach 10% of its initial value (in minutes)?
a.) 256 b.) 556 c.) 1156 d.) 1356
Easy right? Let me know your answers in the comments and I will tell you whether your answer is correct. I hope you learned something from this tutorial. See you soon!