The Black Queen Explains Reductive Evolution
Genetically-wimpy bacteria are among the most successful free-living microbes on Earth. A new evolutionary theory, The Black Queen Hypothesis, explains why.
Complexity is not the inevitable result of evolution say J. Jeffrey Morris and Richard E. Lenski at Michigan State University (MSU) and Erik R. Zinser at the University of Tennessee in Knoxville. Highly successful Prochlorococcus mutants, for example, long ago jettisoned a costly but critically important survival gene and swept their more genetically encumbered ancestors out of the oceans. Why such reductive evolution happens is explained by the group's newly devised Black Queen Hypothesis (BQH).
source
"The Black Queen here refers to the Queen of Spades, which players in the game of Hearts try to get rid of because it's so costly," Lenski notes. From the evolutionary perspective, certain genes and the biological functions they encode are similarly costly and thus undesirable; losing them, therefore, provides a fitness advantage. The BQH explains how genetically-wimpy bacteria, those unlikely to survive even an hour on their own in the wild, have become some of the successful free-living bacteria on Earth. It also shows why genes that encode essential functions are surprisingly rare in some microbial communities.
Shedding light on shedding genes
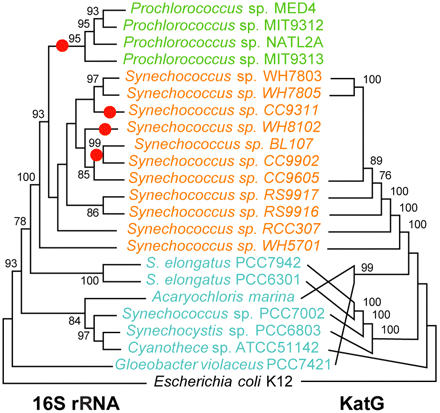
The gene lost to modern-day Prochlorococcus is katG, which protects against hydrogen peroxide, a toxic byproduct of organic carbon photooxidation. Sterile, filtered sea water exposed to sunlight generates enough hydrogen peroxide in a few hours to kill cultured modern-day katG-deficient Prochlorococcus; thus being defenseless against it in the wild should prove fatal --yet it isn't, say these researchers. Moreover, the loss of katG in Prochlorococcus was likely driven by selection, says Morris, because less genetic baggage reduces the amount of energy or nutrients needed, all of which are in short supply in the open ocean.
Importantly, an organism having its genome streamlined must be able to do without the lost genes in its natural environment, Zinser stresses. "This is possible for Prochlorococcus because other bacteria within its community protect their own interiors with katG-encoded catalase-peroxidase --a primary defense against hydrogen peroxide in cyanobacteria-- and enough peroxidase activity 'leaks' out to protect all the cells in their immediate vicinity. In this way, some marine microbes act as unintentional 'helpers,' protecting the vulnerable majority --the 'beneficiaries-- as a side effect of helping themselves," he explains.
Furthermore, even though protecting their neighbors is neither altruistic nor self-enriching according to Morris, the helper cells can also benefit from the association. For example,their helpers probably depend on Prochlorococcus for carbon, so if genome reduction enables Prochlorococcus to produce more organic carbon, the helper community also benefits greatly from the relationship. "Thus the association between Prochlorococcus and its helpers isn't merely commensalistic; it's mutualistic," he adds.
The BQH is different than the parasite-host relationship where reductive evolution is common; the "leaky" microbe here is not sacrificing any of its fitness by providing the community with an important "public good." In fact, beyond simply getting more to eat on occasion, helper cells might also be ensuring their own survival by becoming indispensible members of their microbial community.
Costly public goods that can leak in and out of cells are pivotal features of the BQH
"Any function that is both leaky and costly to perform is a potential target for gene loss," says Lenski. Inorganic nutrient acquisition, nitrogen fixation, and biofilm matrix deposition meet these criteria and are currently being investigated within the framework of the BQH. Preliminary studies have recently shown that another abundant, marine bacterium "Ca.Pelagibacter ubique" has lost the genes required for sulfate reduction and that this streamlining is possible because it gets all the dimethylsulfoniopropionate (DMSP) it needs from a diverse group of phytoplankton helpers.
"In contrast to other theories of coevolution, the relationship between helpers and beneficiaries doesn't require direct interaction, but happens instead because the beneficiary can simply stop performing a costly function owing to the common goods provided by a 'leaky' helper," says Morris. It also shows that individual-level selection in microbial communities, like those in human and other social animals, can lead to a division of labor that becomes advantageous to all.
Richard Losick of Harvard University, who edited the mBio paper, says "It's a sweeping hypothesis for how free-living microorganisms evolve to become dependent on each other," which, he adds "…offers a new way of looking at [their] complicated, inter-dependent communities….."
Anton Post from the Marine Biological Laboratory (MBL) in Woods Hole, Massachusetts notes that "This elegant hypothesis touches on the well known ecological concepts of commensalism, mutualism or parasitism and provides a framework to test some underlying assumptions about their evolution in microbial communities."
References:
- Richard Lenski: Experimental Evolution http://myxo.css.msu.edu/ResearchInterests.html
- The University of Tennessee, Knoxville https://micro.utk.edu/faculty/zinser.php
- Morris JJ, Lenski RE, Zinser ER: The Black Queen Hypothesis: Evolution of Dependencies through Adaptive Gene Loss https://mbio.asm.org/content/3/2/e00036-12
As a follower of @followforupvotes this post has been randomly selected and upvoted! Enjoy your upvote and have a great day!
This post has been voted on by the SteemSTEM curation team and voting trail in collaboration with @curie.
If you appreciate the work we are doing then consider voting both projects for witness by selecting stem.witness and curie!
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!